Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial -

Tranexamic acid for prevention of hemorrhage in elective repeat cesarean delivery—a randomized study - American Journal of Obstetrics & Gynecology MFM
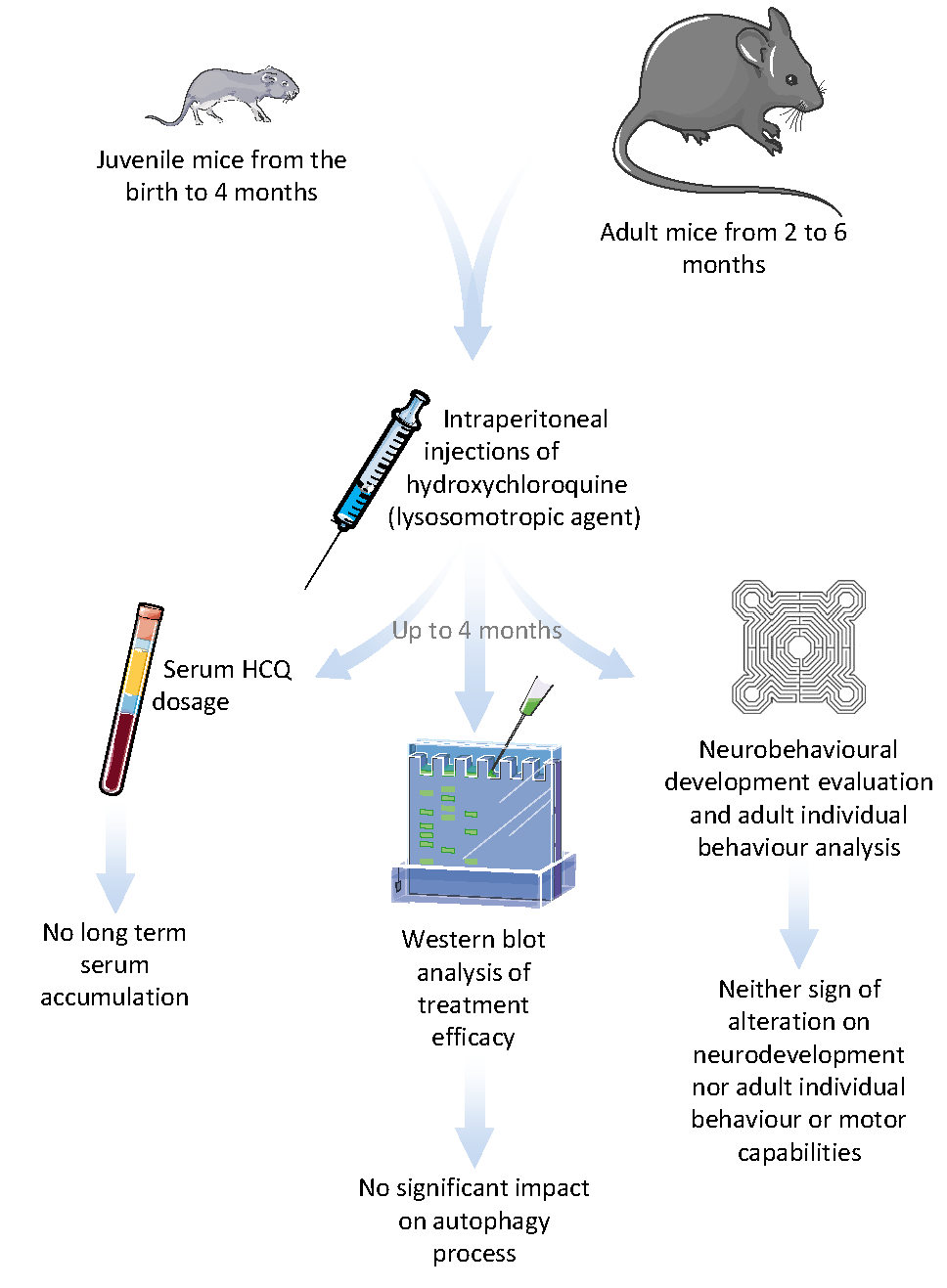
Biomedicines | Free Full-Text | Long Term Pharmacological Perturbation of Autophagy in Mice: Are HCQ Injections a Relevant Choice? | HTML

SARS-CoV-2 Omicron-neutralizing memory B cells are elicited by two doses of BNT162b2 mRNA vaccine | Science Immunology

Safety and Immunogenicity of MVC-COV1901 Vaccine in Older Adults: Phase 2 Randomized Dose-Comparison Trial - International Journal of Infectious Diseases

Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19 | NEJM

Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells | Science

An AAV-based, room-temperature-stable, single-dose COVID-19 vaccine provides durable immunogenicity and protection in non-human primates - ScienceDirect
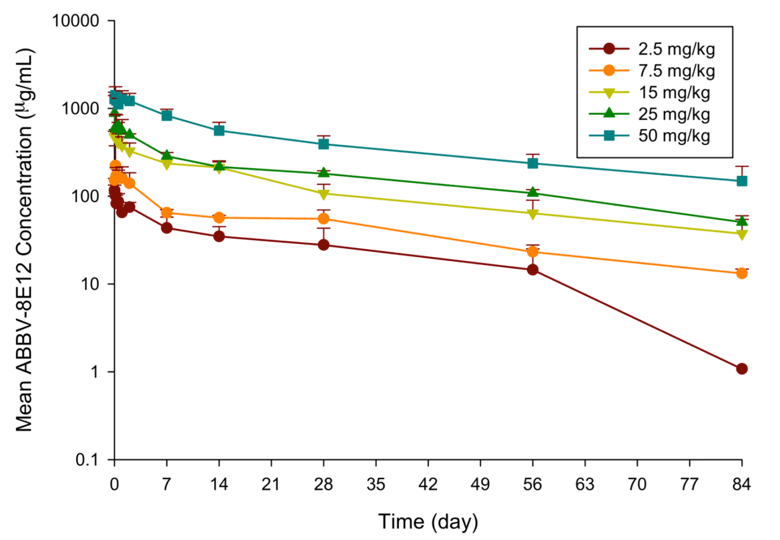
PRECLINICAL AND CLINICAL DEVELOPMENT OF ABBV-8E12, A HUMANIZED ANTI-TAU ANTIBODY, FOR TREATMENT OF ALZHEIMER'S DISEASE AND OTHER TAUOPATHIES • The Journal of Prevention of Alzheimer's Disease

Omecamtiv mecarbil does not prolong QTc intervals at therapeutic concentrations - Trivedi - - British Journal of Clinical Pharmacology - Wiley Online Library
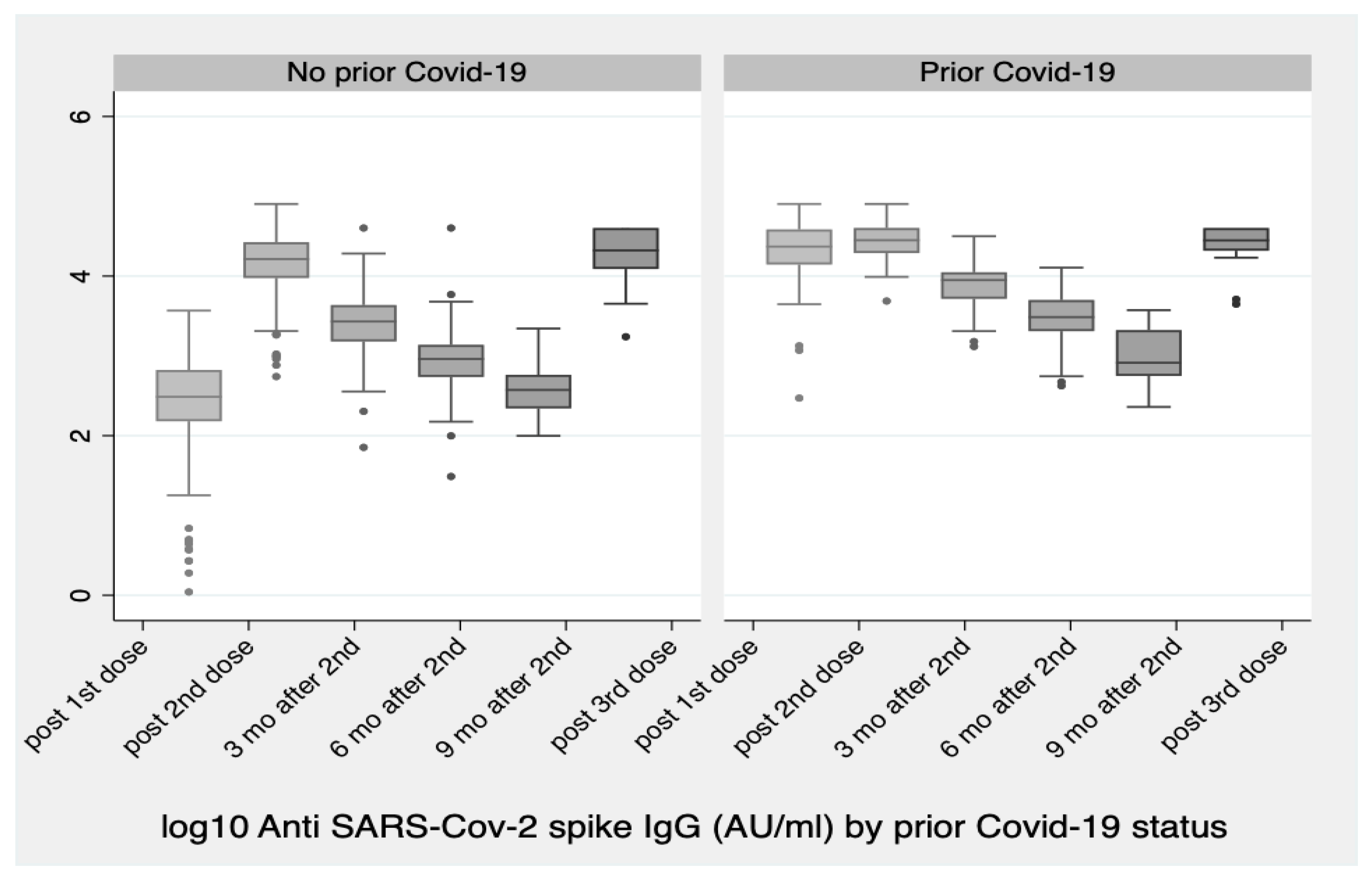
Vaccines | Free Full-Text | Significant Increase in Antibody Titers after the 3rd Booster Dose of the Pfizer–BioNTech mRNA COVID-19 Vaccine in Healthcare Workers in Greece | HTML

mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection | Science

Robust immune responses are observed after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2–experienced individuals | Science Translational Medicine

Robust immune responses are observed after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2–experienced individuals | Science Translational Medicine
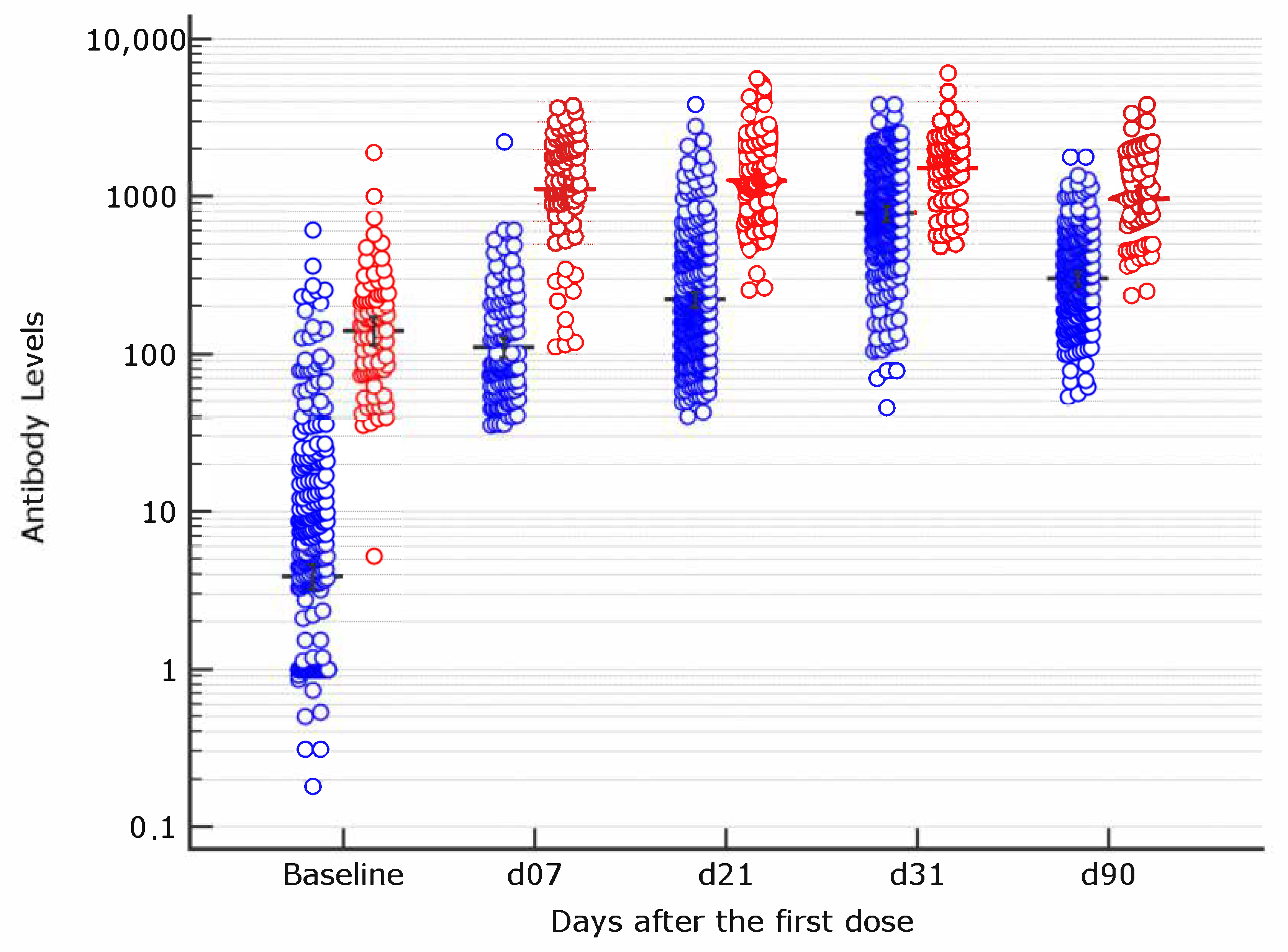
Vaccines | Free Full-Text | Early Serological Response to BNT162b2 mRNA Vaccine in Healthcare Workers | HTML

TropicalMed | Free Full-Text | Humoral Immune Response Induced by the BBIBP-CorV Vaccine (Sinopharm) in Healthcare Workers: A Cohort Study | HTML

Robust immune responses are observed after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2–experienced individuals | Science Translational Medicine
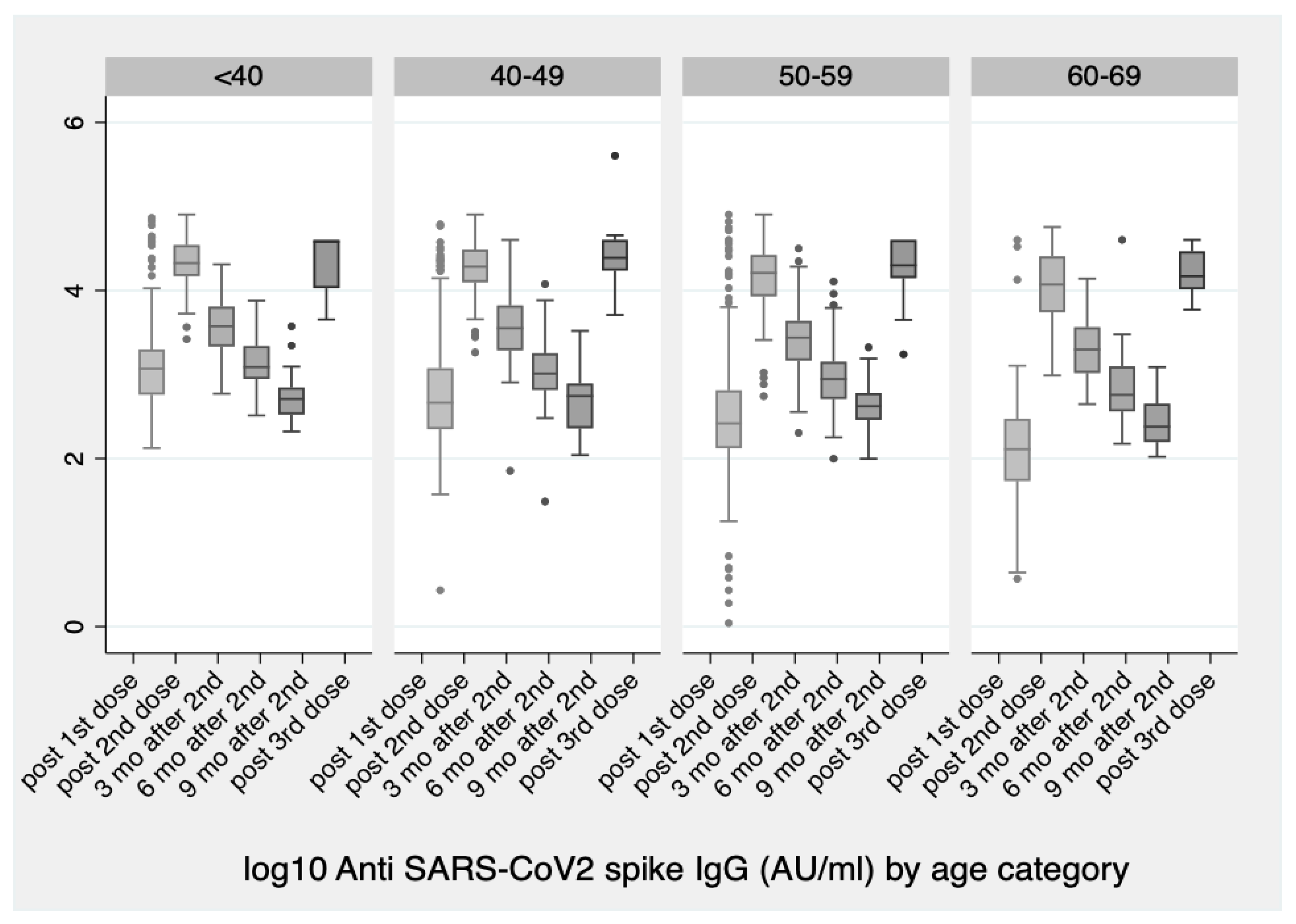
Vaccines | Free Full-Text | Significant Increase in Antibody Titers after the 3rd Booster Dose of the Pfizer–BioNTech mRNA COVID-19 Vaccine in Healthcare Workers in Greece | HTML
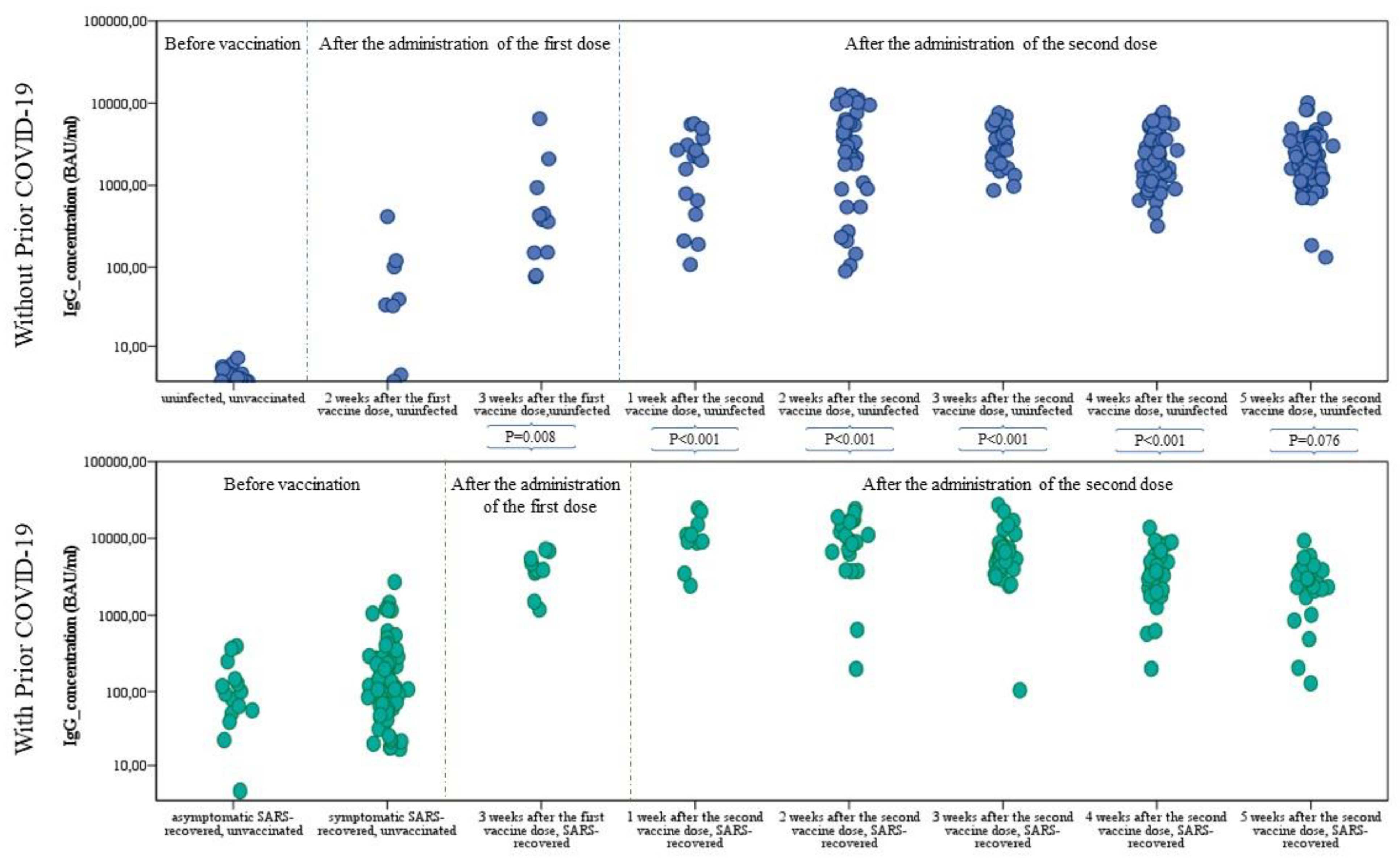
Cells | Free Full-Text | Differences in the Concentration of Anti-SARS-CoV-2 IgG Antibodies Post-COVID-19 Recovery or Post-Vaccination | HTML

Two doses of the SARS-CoV-2 BNT162b2 vaccine enhance antibody responses to variants in individuals with prior SARS-CoV-2 infection | Science Translational Medicine

Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial -