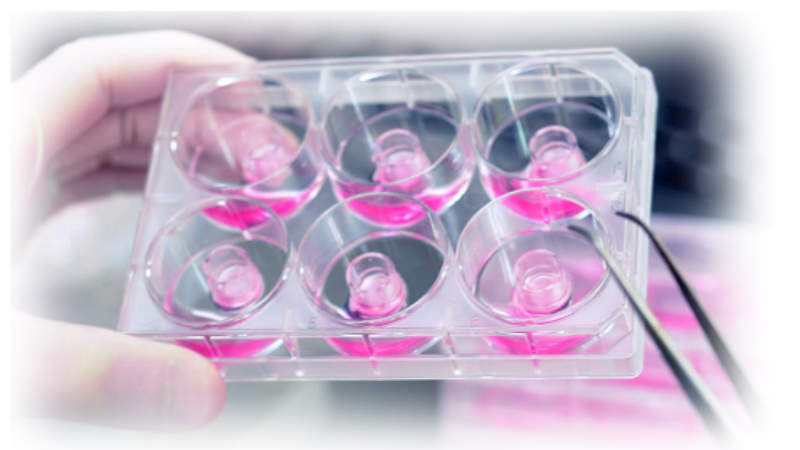
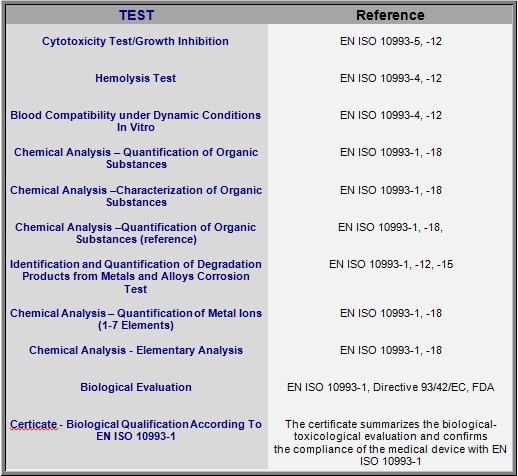
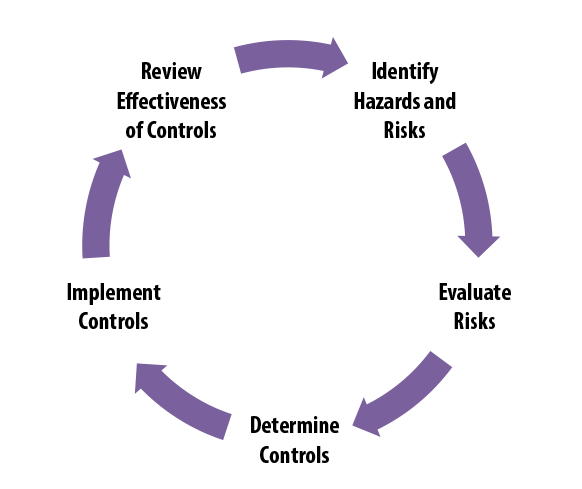
Biological Evaluation Plan and Report - MakroCareRegulatory, Clinical Consulting Services to Biopharma & Medical Device Companies | MakroCare

Biological Evaluation Plan: A crucial first step in the Biocompatibility evaluation of a Med Device - YouTube